Introduction
Inflammation in the periapical area of the periodontal ligament is similar to that occurring elsewhere in the body. It is often accompanied by resorption of bone, and occasionally the root apex, sufficient to be detected radiographically. However, the periapical vascular network has a rich collateral circulation, greatly enhancing the ability of the tissue to heal if the cause of the inflammation is removed. This potential for complete periapical healing, providing the source of irritation is removed, is the basis of endodontic treatment.
Whether the response to irritation in the periodontal ligament is principally an acute or chronic inflammation depends on factors such as the number and virulence of any microorganisms involved, the type and severity of any mechanical or chemical irritant, and the efficiency of the host defences. While it is convenient to describe acute and chronic periapical periodontitis as separate conditions, it must be realized that the tissue reaction to irritation is a dynamic response, often vacillating with time between acute and chronic inflammation. The sequelae are determined by the balance between the nature, severity, and duration of the irritant and the integrity of the defence mechanisms of the patient.
Aetiology
Introduction
The main causes of periapical periodontitis are detailed below.
Pulpitis and pulp necrosis
If pulpitis is untreated bacteria, bacterial toxins, or the products of inflammation will in time extend down the root canal and through the apical foramina to cause periodontitis. When pulp necrosis follows other causes, for example a blow to the tooth damaging the apical vessels, clinically significant periodontitis does not develop, unless bacteria gain access to the necrotic pulp or to the periapical tissues. The possible changes that may occur around the apex of an infected non-vital tooth and their inter-relationships are illustrated in
Changes that may occur around the
apex of an infected non-vital tooth
Trauma
Occlusal trauma from, for example, a high restoration, undue pressure during orthodontic treatment, a direct blow on a tooth, and biting unexpectedly on a hard body in food may all cause minor damage to the periodontal ligament and localized inflammation. Traumatic periodontitis is often acute and transitory.
Key points - Periapical periodontitis
dynamic process; inflammation can vary with time
outcome reflects the balance between the nature, duration, and severity of the irritant and the effectiveness of the host defences
bacterial infection of the root canals is the major cause of clinically significant periodontitis
can follow acute traumatic injury to periapical tissues without pulp necrosis; usually transient
Key points - Mnemonic for differential diagnosis of pain of pulpal and periapical origin - LOCATE
Location
Other symptoms
Character
Associations
Timing
Evaluation of other investigations, e.g. pulp vitality tests
Occlusal trauma from, for example, a high restoration, undue pressure during orthodontic treatment, a direct blow on a tooth, and biting unexpectedly on a hard body in food may all cause minor damage to the periodontal ligament and localized inflammation. Traumatic periodontitis is often acute and transitory.
Key points - Periapical periodontitis
dynamic process; inflammation can vary with time
outcome reflects the balance between the nature, duration, and severity of the irritant and the effectiveness of the host defences
bacterial infection of the root canals is the major cause of clinically significant periodontitis
can follow acute traumatic injury to periapical tissues without pulp necrosis; usually transient
Key points - Mnemonic for differential diagnosis of pain of pulpal and periapical origin - LOCATE
Location
Other symptoms
Character
Associations
Timing
Evaluation of other investigations, e.g. pulp vitality tests
Endodontic treatment
Mechanical instrumentation through the root apex during endodontic treatment, as well as chemical irritation from root-filling materials, may result in inflammation in the periapical periodontium. Instrumentation of an infected root canal may also be followed by periapical inflammation as a result of bacteria being forced inadvertently into the periapical tissues.
Acute periapical periodontitis
This is characterized by an acute inflammatory exudate in the periodontal ligament within the confined space between the root apex and the alveolar bone. Pain is elicited when external pressure is applied to the tooth because the pressure is transmitted through the fluid exudate to the sensory nerve endings. Even light touch may be sufficient to induce pain and, unlike pulpitis, this is generally well located by the patient to a particular tooth due to stimulation of proprioceptive nerve endings in the periodontal ligament. As the fluid is not compressible, the tooth feels elevated in its socket. Hot or cold stimulation of the tooth does not cause pain, as it would in pulpitis. The radiographic appearances are often normal as there is generally insufficient time for bone resorption to occur between the time of injury to the periodontal ligament and the onset of symptoms. If radiological changes are present, they consist of slight widening of the periodontal ligament and the lamina dura around the apex may be less well defined than normal.
The inflammation may be transient if it is due to acute trauma rather than infection and the condition soon resolves. If the irritant persists the inflammation becomes chronic and may be associated with resorption of the surrounding bone. Suppuration may occur if there is severe irritation and tissue necrosis associated with bacterial infection and the continued and massive exudation of neutrophil leucocytes leading to abscess formation. Such an abscess is called an acute periapical or alveolar abscess and, although such abscesses may develop directly from acute apical periodonitis, most arise because of acute exacerbation within a pre-existing periapical granuloma (see below and).
Mechanical instrumentation through the root apex during endodontic treatment, as well as chemical irritation from root-filling materials, may result in inflammation in the periapical periodontium. Instrumentation of an infected root canal may also be followed by periapical inflammation as a result of bacteria being forced inadvertently into the periapical tissues.
Acute periapical periodontitis
This is characterized by an acute inflammatory exudate in the periodontal ligament within the confined space between the root apex and the alveolar bone. Pain is elicited when external pressure is applied to the tooth because the pressure is transmitted through the fluid exudate to the sensory nerve endings. Even light touch may be sufficient to induce pain and, unlike pulpitis, this is generally well located by the patient to a particular tooth due to stimulation of proprioceptive nerve endings in the periodontal ligament. As the fluid is not compressible, the tooth feels elevated in its socket. Hot or cold stimulation of the tooth does not cause pain, as it would in pulpitis. The radiographic appearances are often normal as there is generally insufficient time for bone resorption to occur between the time of injury to the periodontal ligament and the onset of symptoms. If radiological changes are present, they consist of slight widening of the periodontal ligament and the lamina dura around the apex may be less well defined than normal.
The inflammation may be transient if it is due to acute trauma rather than infection and the condition soon resolves. If the irritant persists the inflammation becomes chronic and may be associated with resorption of the surrounding bone. Suppuration may occur if there is severe irritation and tissue necrosis associated with bacterial infection and the continued and massive exudation of neutrophil leucocytes leading to abscess formation. Such an abscess is called an acute periapical or alveolar abscess and, although such abscesses may develop directly from acute apical periodonitis, most arise because of acute exacerbation within a pre-existing periapical granuloma (see below and).
Periapical granuloma with central zone of suppurative inflammation
Chronic
periapical periodontitis (periapical or apical granuloma)
Introduction
Persistent irritation, usually derived from bacteria and their products in the pulp chamber and root canals, leads to chronic periapical periodontitis. This is characterized by resorption of the periapical alveolar bone and its replacement by chronically inflamed granulation tissue to form a periapical granuloma. Around the periphery of the lesion the chronic inflammatory stimuli may lead to the formation of dense bundles of collagen fibres that separate the chronically inflamed granulation tissue from the surrounding bone. These collagen fibres, forming a sort of capsule around the lesion, are attached to the root surface and in some cases the granuloma may be removed attached to the extracted toot .
Histologically the lesion consists mainly of granulation tissue infiltrated by lymphocytes, plasma cells, and macrophages and, although the composition of the inflammatory infiltrate varies considerably, T-lymphocytes predominate. Immunological reactions, in response to persistent antigenic stimulation derived from the pulp chamber and root canals, are key factors in the development of the lesion. In addition to the inflammatory infiltrate, deposits of cholesterol and haemosiderin are often present in a periapical granuloma and both are probably derived from the breakdown of extravasated red blood cells. Cholesterol crystals in the granulation tissue are represented in routine histological sections as empty needle-like spaces or clefts, the crystals having dissolved out in the reagents used in section preparation. Multinucleate foreign-body giant cells are grouped around the cholesterol clefts. Foci of lipid-laden macrophages - foam cells - may also be seen . Epithelial cell rests of Malassez incorporated within the granuloma may begin to proliferate, probably as a result of stimulation by growth factors released by a variety of cells within the granuloma. The proliferated squamous epithelium forms anastomosing cords, often arranged in loops or arcades, throughout the granulation tissue. Neutrophil leucocytes in varying stages of degeneration are often seen infiltrating the oedematous intercellular spaces of the epithelium.
Periapical granulomas tend to be asymptomatic, but may be associated with occasional tenderness of the tooth to palpation and percussion. Percussion may produce a dull note because of the lack of resonance caused by the granulation tissue around the apex. Radiological examination at first shows a widening of the periodonal ligament space around the apex and later a definite periapical radiolucency may develop. In some instances this radiolucency is well circumscribed and clearly demarcated from the surrounding bone by a corticated margin, while in others the border is poorly defined. These appearances are related to differences in cellular activity around the margins of the lesion. Where there is active bone resorption and expansion of the lesion the margin is ill-defined. Where the lesion is static and a balance is established between the level of irritation and the host defences, the chronic inflammatory stimulus may lead to bone apposition and the formation of a zone of sclerosis around the lesion (see osteosclerosis (point 5) below). Histological evidence of external resorption of the apical cementum and dentine is frequent and is occasionally sufficient to be detected radiographically.
The importance of the root canal as a continued source of infection and antigenic challenge in an apical granuloma is shown by the fact that most periapical lesions heal once the canal is sealed by satisfactory endodontic treatment. The predominant organisms are obligate anaerobes (70 per cent or more) with smaller numbers of facultative anaerobes. Microorganisms surviving in the root canals or dentinal tubules after endodontic treatment may be important in teeth with persistent apical radiolucencies.
Introduction
Persistent irritation, usually derived from bacteria and their products in the pulp chamber and root canals, leads to chronic periapical periodontitis. This is characterized by resorption of the periapical alveolar bone and its replacement by chronically inflamed granulation tissue to form a periapical granuloma. Around the periphery of the lesion the chronic inflammatory stimuli may lead to the formation of dense bundles of collagen fibres that separate the chronically inflamed granulation tissue from the surrounding bone. These collagen fibres, forming a sort of capsule around the lesion, are attached to the root surface and in some cases the granuloma may be removed attached to the extracted toot .
Histologically the lesion consists mainly of granulation tissue infiltrated by lymphocytes, plasma cells, and macrophages and, although the composition of the inflammatory infiltrate varies considerably, T-lymphocytes predominate. Immunological reactions, in response to persistent antigenic stimulation derived from the pulp chamber and root canals, are key factors in the development of the lesion. In addition to the inflammatory infiltrate, deposits of cholesterol and haemosiderin are often present in a periapical granuloma and both are probably derived from the breakdown of extravasated red blood cells. Cholesterol crystals in the granulation tissue are represented in routine histological sections as empty needle-like spaces or clefts, the crystals having dissolved out in the reagents used in section preparation. Multinucleate foreign-body giant cells are grouped around the cholesterol clefts. Foci of lipid-laden macrophages - foam cells - may also be seen . Epithelial cell rests of Malassez incorporated within the granuloma may begin to proliferate, probably as a result of stimulation by growth factors released by a variety of cells within the granuloma. The proliferated squamous epithelium forms anastomosing cords, often arranged in loops or arcades, throughout the granulation tissue. Neutrophil leucocytes in varying stages of degeneration are often seen infiltrating the oedematous intercellular spaces of the epithelium.
Periapical granulomas tend to be asymptomatic, but may be associated with occasional tenderness of the tooth to palpation and percussion. Percussion may produce a dull note because of the lack of resonance caused by the granulation tissue around the apex. Radiological examination at first shows a widening of the periodonal ligament space around the apex and later a definite periapical radiolucency may develop. In some instances this radiolucency is well circumscribed and clearly demarcated from the surrounding bone by a corticated margin, while in others the border is poorly defined. These appearances are related to differences in cellular activity around the margins of the lesion. Where there is active bone resorption and expansion of the lesion the margin is ill-defined. Where the lesion is static and a balance is established between the level of irritation and the host defences, the chronic inflammatory stimulus may lead to bone apposition and the formation of a zone of sclerosis around the lesion (see osteosclerosis (point 5) below). Histological evidence of external resorption of the apical cementum and dentine is frequent and is occasionally sufficient to be detected radiographically.
The importance of the root canal as a continued source of infection and antigenic challenge in an apical granuloma is shown by the fact that most periapical lesions heal once the canal is sealed by satisfactory endodontic treatment. The predominant organisms are obligate anaerobes (70 per cent or more) with smaller numbers of facultative anaerobes. Microorganisms surviving in the root canals or dentinal tubules after endodontic treatment may be important in teeth with persistent apical radiolucencies.
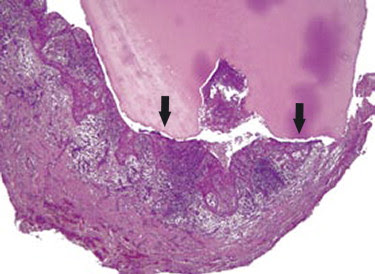
Periapical
granuloma attached to extracted root.
|
||
Histological section of root and attached periapical granuloma from.Note the more
heavily inflamed central area of the periapical granuloma (blue/purple
stained), compared to the less inflamed, more collagenous peripheral zone.
|
||
|
|
||
Periapical
granuloma containing proliferating strands of squamous epithelium.
|
|
Periapical
radiolucency and apical resorption associated with a periapical granuloma.
|
Sequelae
1. If the level of antigenic challenge is in equilibrium with the host's immunological response, the granuloma can remain quiescent for long periods. However, if the equilibrium is disturbed in favour of the microbial flora in the root canal, the granuloma will continue to enlarge and be associated with continued resorption of bone, the process being symptomless, until equilibrium is restored.
2. When organisms invade the granuloma from the root canal acute exacerbation is likely and the patient may present with acute symptoms. Acute exacerbation can cause rapid enlargement of the lesion and may progress to abscess formation (see below). Alternatively, the inflammatory response may overcome the infection and a new equilibrium can be established.
3. Suppuration may occur in the granuloma. This may continue to enlarge to form an acute periapical (alveolar) abscess. Clinically this may present with rapid onset of pain, followed by redness and swelling of the adjacent soft tissues as the abscess tracks and points. The affected tooth is tender to percussion, and there may be slight mobility on palpation. Alternatively, the area of suppuration may be contained by the host's defences to form a chronic abscess that shows little tendency to enlarge or spread and which causes few, if any, clinical signs or symptoms until a further acute exacerbation.
4. Proliferation of the epithelial cell rests of Malassez associated with the inflammation may lead to the development of an inflammatory radicular cyst.
5. Low-grade irritation to the apical tissues may result in bone apposition (osteosclerosis) rather than resorption, histologically a mild chronic inflammatory infiltrate being seen in the rather scanty, fibrous marrow. The process is clinically asymptomatic and shows as an opaque area of bone on radiographs. On occasions, the opacity is well circumscribed while on others it shows no clear line of demarcation from the normal surrounding bone.
6. Low-grade irritation to the apical tissues may also result in the apposition of cementum on the adjacent root surface to produce hypercementosis.
Key points - Periapical granuloma
chronically inflamed granulation tissue around apex of a non-vital tooth
infection and antigenic challenge from endodontic flora
apical radiolucency; margins reflect dynamics of the lesion
host response may be in equilibrium with level of irritation
may be symptomless and remain quiescent for long periods
stimulation and proliferation of rests of Malassez within the lesion
1. If the level of antigenic challenge is in equilibrium with the host's immunological response, the granuloma can remain quiescent for long periods. However, if the equilibrium is disturbed in favour of the microbial flora in the root canal, the granuloma will continue to enlarge and be associated with continued resorption of bone, the process being symptomless, until equilibrium is restored.
2. When organisms invade the granuloma from the root canal acute exacerbation is likely and the patient may present with acute symptoms. Acute exacerbation can cause rapid enlargement of the lesion and may progress to abscess formation (see below). Alternatively, the inflammatory response may overcome the infection and a new equilibrium can be established.
3. Suppuration may occur in the granuloma. This may continue to enlarge to form an acute periapical (alveolar) abscess. Clinically this may present with rapid onset of pain, followed by redness and swelling of the adjacent soft tissues as the abscess tracks and points. The affected tooth is tender to percussion, and there may be slight mobility on palpation. Alternatively, the area of suppuration may be contained by the host's defences to form a chronic abscess that shows little tendency to enlarge or spread and which causes few, if any, clinical signs or symptoms until a further acute exacerbation.
4. Proliferation of the epithelial cell rests of Malassez associated with the inflammation may lead to the development of an inflammatory radicular cyst.
5. Low-grade irritation to the apical tissues may result in bone apposition (osteosclerosis) rather than resorption, histologically a mild chronic inflammatory infiltrate being seen in the rather scanty, fibrous marrow. The process is clinically asymptomatic and shows as an opaque area of bone on radiographs. On occasions, the opacity is well circumscribed while on others it shows no clear line of demarcation from the normal surrounding bone.
6. Low-grade irritation to the apical tissues may also result in the apposition of cementum on the adjacent root surface to produce hypercementosis.
Key points - Periapical granuloma
chronically inflamed granulation tissue around apex of a non-vital tooth
infection and antigenic challenge from endodontic flora
apical radiolucency; margins reflect dynamics of the lesion
host response may be in equilibrium with level of irritation
may be symptomless and remain quiescent for long periods
stimulation and proliferation of rests of Malassez within the lesion
Osteosclerosis around the roots of a mandibular molar.