OSTEOMYELITIS
- Suppurative osteomyelitis
- Garrés osteomyelitis (periostitis ossificans, proliferative osteomyelitis)
- Tuberculous osteomyelitis
- Syphilitic osteomyelitis
- Actinomycotic osteomyelitis
- Osteoradionecrosis
SUPPURATIVE OSTEOMYELITIS
Osteomyelitis is an inflammatory reaction of bone to
infection which originates from either a tooth, fracture site, soft tissue
wound or surgery site. The dental infection may be from a root canal, a
periodontal ligament or an extraction site. Suppurative osteomyelitis can involve
all three components of bone: periosteum, cortex, and marrow. Usually there is
an underlying predisposing factor like malnutrition, alcoholism, diabetes,
leukemia or anemia.
Other predisposing factors are those that are characterized
by the formation of avascular bone for example, therapeutically irradiated
bone, osteopetrosis, Paget's disease, and florid osseous dysplasia.
Osteomyelitis is more commonly observed in the mandible because of its poor
blood supply as compared to the maxilla, and also because the dense mandibular
cortical bone is more prone to damage and, therefore, to infection at the time
of tooth extraction.
Acute osteomyelitis is similar to an acute primary abscess
in that the onset and course may be so rapid that bone resorption does not
occur and, thus, a radiolucency may not be present on a radiograph. Clinical
features include pain, pyrexia, painful lymphadenopathy, leukocytosis, and
other signs and symptoms of acute infection. Later, after approximately two
weeks, as the lesion progresses into the chronic stage, enough bone resorption
takes place to show radiographic mottling and blurring of bone. A sclerosed
border called an involucrum forms around the affected area. The involucrum
prevents blood supply from reaching the affected part. This results in the
formation of pieces of sequestra or necrotic bone surrounded by pus. A
fistulous tract may develop by the suppuration perforating the cortical bone
and periosteum. The fistulous tract discharges pus onto the overlying skin or mucosa.
The radiopacity of the sequestra and the radiolucency of the
pus give rise to the characteristic "worm-eaten" radiographic
appearance. Radiographs also aid in locating the original site of infection
such as an infected tooth, a fracture, or infected sinus.
Fig. 13-1 Chronic suppurative osteomyelitis of dental
origin. The lesion discharged pus into the oral cavity. Note the radiopaque
sequestra (arrow) surrounded by the radiolucent suppuration.
Fig. 13-2 Chronic suppurative osteomyelitis demonstrating a
worm-eaten appearance of the body of the mandible. Note the radiopaque
sequestra surrounded by the radiolucent suppuration and a radiopaque
involucrum. The patient had fetid breath.
Fig. 13-3 Chronic suppurative osteomyelitis of dental
origin. The radiopaque sequestrum (arrow) is surrounded by the radiolucent
suppuration.
Fig. 13-4 Sequestrum that has floated into the soft tissues.
Patient gave a history of a problematic tooth extraction several years ago
which resulted in clinical complications.
GARRÉS OSTEOMYELITIS
(Periostitis ossificans, Osteomyelitis with proliferative periostitis)
Garrés osteomyelitis or proliferative periostitis is a type of chronic
osteomyelitis which is nonsuppurative. It occurs almost exclusively in children
and young adults who present symptoms related to a carious tooth. The process
arises secondary to a low-grade chronic infection, usually from the apex of a
carious mandibular first molar. The infection spreads towards the surface of
the bone, resulting in inflammation of the periosteum and deposition of new
bone underneath the periosteum. This peripheral formation of reactive bone
results in localized periosteal thickening. The inferior border of the mandible
below the carious first molar is the most frequent site for the hard nontender
expansion of cortical bone. On an occlusal view radiograph, the deposition of
new bone produces an "onion-skin" appearance.
Fig. 13-5 Garrés osteomyelitis (proliferative periostitis)
demonstrating an expansion of the inferior border of the mandible (onion-skin
appearance) caused by the periapical infection of the mandibular first molar.
Fig. 13-6 An occlusal radiograph of Garrés osteomyelitis
showing the buccal expansion of the mandible caused by infection around the root
tip of the extracted first molar.
Fig. 13-7 Garrés osteomyelitis (periostitis ossificans)
exhibiting localized periosteal thickening. The source of infection is not known;
it could have been from an exfoliated deciduous molar tooth.
TUBERCULOSIS OSTEOMYELITIS
Tuberculosis is a chronic granulomatous disease which may
affect any organ, although in man the lung is the major seat of the disease and
is the usual portal through which infection reaches other organs. The
microorganisms may spread by either the bloodstream or the lymphatics. Oral
manifestations of tuberculosis are extremely rare and are usually secondary to
primary lesions in other parts of the body. Infection of the socket after tooth
extraction can also be the mode of entry into the bone by Mycobacterium
tuberculosis.
Mandible and maxilla are less commonly affected than long
bones and vertebrae. On a radiograph, the appearance of bony lesions is similar
to that of chronic suppurative osteomyelitis ("worm- eaten"
appearance) with fistulae formation through which small sequestra are exuded.
Periostitis ossificans (proliferative periostitis) can also occur and change
the contour of bone. Calcification of lymph nodes is a characteristic sign of tuberculosis.
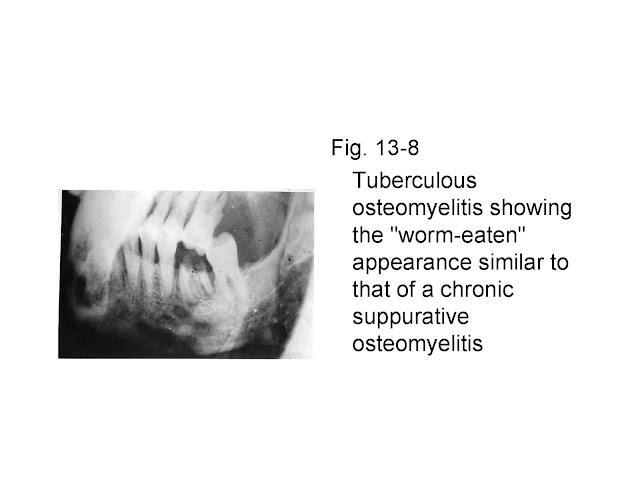
Fig. 13-8 Tuberculous osteomyelitis showing the "worm-eaten"
appearance similar to that of a chronic suppurative osteomyelitis
Fig. 13-9 Calcified tuberculous lymph nodes
SYPHILITIC OSTEOMYELITIS
Syphilis is a chronic granulomatous disease which is caused
by the spirochete Treponema pallidum. It is a contagious venereal disease which
leads to many structural and cutaneous lesions. Acquired syphilis is
transmitted by direct contact whereas congenital syphilis is transmitted in
utero. In congenital syphilis, the teeth are hypoplastic, that is, the
maxillary incisors have screwdriver-shaped crowns with notched incisal edges
(Hutchinson's teeth) and the molars have irregular mass of globules instead of
well-formed cusps ("mulberry molars"). Also, a depressed nasal bridge
or saddleback nose occurs because of gummatous destruction of the nasal bones.
Acquired syphilis, if untreated, has three distinct stages.
The primary stage develops after a couple of weeks of exposure and consists of
chancres on the lips, tongue, palate, oral mucosa, penis, vagina, cervix or anus.
These chancres are contagious on direct contact with them. The secondary stage
begins 5 to 10 weeks after the occurrence of chancres and consists of diffuse
eruptions on skin and mucous membrane. This rash may be accompanied by swollen
lymph nodes throughout the body, a sore throat, weight loss, malaise, headache
and loss of hair. The secondary stage can also damage the eyes, liver, kidneys
and other organs. The tertiary-stage lesions may not appear for several years
to decades after the onset of the disease. In this stage of osteomyelitis, the
bone, skin, mucous membrane, and liver show gummatous destruction which is a
soft, gummy tumor that resembles granulation tissue. Paralysis and dementia can
also occur. In the oral cavity, the hard palate is frequently involved
resulting in its perforation. The gummatous destruction is painless. Syphilitic
osteomyelitis of the jaws is difficult to distinguish from chronic suppurative
osteomyelitis since their radiographic appearances are similar.
Fig.13-10 Syphilitic osteomyelitis of the palate. The
gummatous destruction has produced a palatal perforation.
Fig.13-11 Radiograph of syphilitic osteomyelitis of the
palate. The perforation which is the site of gumma of the hard palate produces
a radiolucency which may be mistaken for a median palatine cyst.
ACTINOMYCOTIC OSTEOMYELITIS
Like tuberculosis and syphilis, actinomycosis is a chronic
granulomatous disease. It can occur anywhere in the body, but two-thirds of all
cases occur in the cervicofacial region. The disease is caused by bacteria-like
fungus called Actinomyces israeli. These microorganisms occur as normal flora
of the oral cavity, and appear to become pathogenic only after entrance through
previously seated defects. The portal of entry for the microorganisms is either
through the socket of an extracted tooth, a traumatized mucous membrane, a
periodontal pocket, the pulp of a carious tooth or a fracture. In cervicofacial
actinomycosis, the patient exhibits swelling, pain, fever and trismus. The lesion
may remain localized in the soft tissues or invade the jaw bones. If the lesion
progresses slowly, little suppuration takes place; however, if it breaks down,
abscesses are formed that discharge pus containing yellow granules ( (nicknamed
sulfur granules) through multiple sinuses.
There is no characteristic radiographic appearance. In some
cases the lesion resembles a periapical radiolucent lesion. The more aggressive
lesion resembles chronic suppurative osteomyelitis. In chronic suppurative
osteomyelitis there is usually a single sinus through which pus exudes;
however, in actinomycotic osteomyelitis there are many sinuses through which
pus and "sulfur granules" exude.
Fig.13-12 Actinomycotic lesion similar to radicular cyst.
This is not a typical appearance.
OSTEORADIONECROSIS (and
effects of irradiation on developing teeth)
In therapeutic radiation for carcinomas of the head and
neck, the jaws are subjected to high exposure doses of ionizing radiation
(average of 5000 R). This results in decreased vascularity of bone and makes
them susceptible to infection and traumatic injury. Infection may occur in
irradiated bone from poor oral hygiene, extraction wound, periodontitis, denture
sores, pulpal infection or dental treatment. It is therefore advisable that a
patient scheduled to undergo therapeutic radiation be given dental treatment
prior to radiation therapy and that after radiation therapy the patient be
taught to maintain good oral hygiene.
When infection occurs in irradiated bone, it results in a
condition called osteoradionecrosis which is similar to chronic suppurative
osteomyelitis. The mandible is affected more commonly than the more vascular
maxilla. Therapeutic radiation may affect the salivary glands, producing
decreased salivation. The resulting temporary or permanent xerostomia is
responsible for radiation caries of teeth and erythema of the mucosa.
A radiograph of osteoradionecrosis, shows radiopaque
sequestra and surrounding radiolucent purulency similar to that of chronic
suppurative osteomyelitis. The two cannot be differentiated radiographically
except by the history of therapeutic radiation. Effects of irradiation on
developing teeth depends on the stage of development when irradiation occurs
and on the dosage administered. The injured tooth germs may either fail to form
teeth (anodontia), exhibit dwarf-teeth, produce agenesis of roots, shortening
and tapering of roots, or develop into hypoplastic teeth. The eruption of teeth
may be retarded and their sequencing may be disturbed. Other radiation induced
effect may include maxillary and/or mandibular hypoplasia.
Fig.13-13 Occlusal projection of anterior region of mandible
showing osteoradionecrosis. Notice the destruction of the trabecular pattern of
bone.
Fig.13-16 Dwarfing of teeth as a consequence of radiation
therapy